
Aug 1, 2016 | In the News
Medical Design & Outsourcing featured MTD in their July issue cover story Molding Manufacturers: Standing out in a crowd We joined companies like MRPC, Sil-Pro, Freudenberg, Moore Med Tech, and Nypro to discuss how to stand out in the crowd, with a...

Aug 1, 2016 | In the News
If you’re looking for insight into the future of the plastics industry, the Plastics News Rising Stars special report are an excellent place to start. This is the publication’s third year of Rising Stars, and the quality continues to shine. The criteria is...
Jul 12, 2016 | In the News
The recent FDA approval of Abbott’s Absorb GT1 Bioresorbable Vascular Scaffold (BVS) System is designed with a poly(L-lactide) polymer to fully dissolve in the patient’s body over about three years. Products like this underscore the growing popularity of...

Jun 28, 2016 | In the News
The MedAccred Plastics Task Group has released its first set of audit criteria. AC8160 MedAccred Audit Criteria for Injection Molding will be used during MedAccred audits conducted at companies manufacturing resin based components via various processes including...
Jun 21, 2016 | In the News
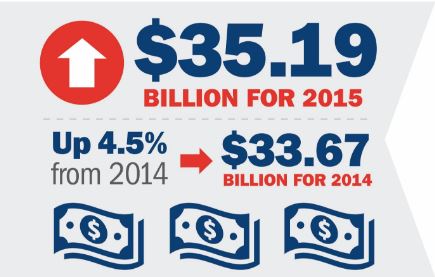
Last week, Plastics News published the Top 100 North American injection molders. Yesterday, Plastics News published data on an additional 467 molders, ranked by North American-related sales, and MTD has made the list. It is great news for the industry that most...

Jun 13, 2016 | In the News
MTD was included in MPO’s June issue in the collaboration article, The Dynamics of Medical Molding. It is always interesting to see different perspectives on different topics in the fast-moving medical device industry. Experts in the field were interviewed on...






