
Jun 28, 2016 | In the News
The MedAccred Plastics Task Group has released its first set of audit criteria. AC8160 MedAccred Audit Criteria for Injection Molding will be used during MedAccred audits conducted at companies manufacturing resin based components via various processes including...

Jun 27, 2016 | Press Release
CHARLTON, MA – (June 27, 2016) – MTD Micro Molding, a long-time leader in medical micro-injection molding, announced the appointment of West-Tech Materials as its sales representative organization for California. “I am very excited about our new...
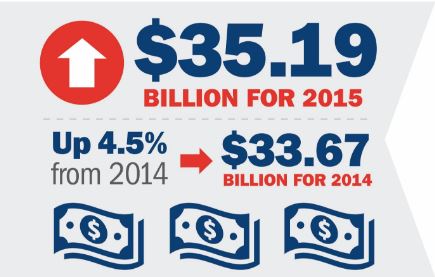
Jun 21, 2016 | In the News
Last week, Plastics News published the Top 100 North American injection molders. Yesterday, Plastics News published data on an additional 467 molders, ranked by North American-related sales, and MTD has made the list. It is great news for the industry that most...

Jun 13, 2016 | In the News
MTD was included in MPO’s June issue in the collaboration article, The Dynamics of Medical Molding. It is always interesting to see different perspectives on different topics in the fast-moving medical device industry. Experts in the field were interviewed on...

Jun 6, 2016 | In the News
The smallest parts often carry with them the greatest importance relative to the functionality and safety of your medical device. MTD refers to its micro molding tools — its road map to success, if you will —as the company’s “6 Sciences”. By embracing these steps, you...

Jun 2, 2016 | Micro Insights
1. Find Solutions: The MD&M East expo floor is your central hub to share ideas, challenges, and solutions with almost 10,000 medtech professionals and 1,000 exhibitors. Don’t miss your chance to meet top-notch medical suppliers, showcasing innovative products a...







