Why MTD
MTD Micro Molding is built for high performance in only one area: the micro molding of advanced medical and healthtech products.
Here are a few reasons why MTD may be the right partner for you.
Key Medical Applications
Exclusive Focus on Medical and Healthtech
Other companies dabble in the medical and healthtech space, but MTD focuses entirely on producing these innovative devices and components. This means we commit all of our people, systems, and operations to meet the needs of our medical customers. As a result, we have developed unmatched expertise in the bioabsorbable and implantable markets to support the ever-advancing medical device and healthtech industry. We also can more quickly respond to emerging applications, like advanced drug delivery, vascular therapies, and wearables.
Bioabsorbable Expertise
We Know Materials. And We Know More of Them.
We can suggest polymer materials that are a good fit for your design for the long term—materials that will work now and be easily sustained as you go forward. If you’re working with implants, you know that there are only a few certified materials, and they’re very expensive. But if you can clearly define your application and its intended use, we might be able to recommend a more costeffective material to consider. Our material database provides predictive data on how a specific material will flow in an application, indicating how the material will perform. Regardless of application, bioabsorbable materials are our expertise.
Problem-Solving
Experts in Problem-Solving
20% of our new projects comes to us a “rescues” – or failed attempts be others. Because our tooling and molding are under one roof, our engineers can efficiently resolve problems and challenges. With MTD’s micro medical molding process, quality standards and sophisticated equipment working together, we can help customers push the envelope—allowing predictable outcomes even on breakthrough products that were never produced before.
Validated Production
Validated Production with Faster Speed to Market
Customers have said the biggest benefit in partnering with MTD Micro Molding is our turnaround time and speed to market. MTD Micro Molding produces acceptable product on the first mold sampling 90% of the time.
Our Validation processes are the gating item between development and production. They’re fully documented, very collaborative, and fully customizable for each client and project. Each part’s quality score is then stored with all the process data, providing a high level of traceability for all our micro medical device parts.
Invested in Innovation
We don’t just talk about innovation. We invest in it.
We invest 10% of our annual revenue to innovation. Our internal R&D projects explore what new micro molding capabilities we can create. We also continually invest in new technologies for providing cutting-edge capabilities in micro medical manufacturing. Why? So our customers can achieve new product breakthroughs faster and more cost-effectively.
Portfolio
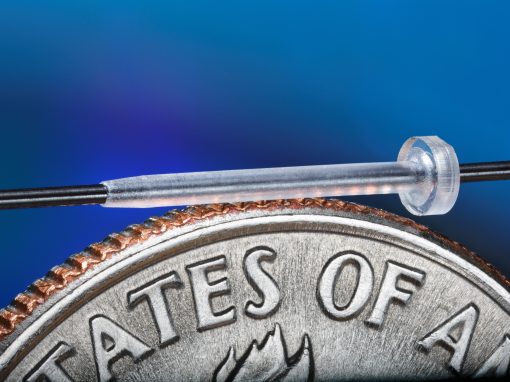
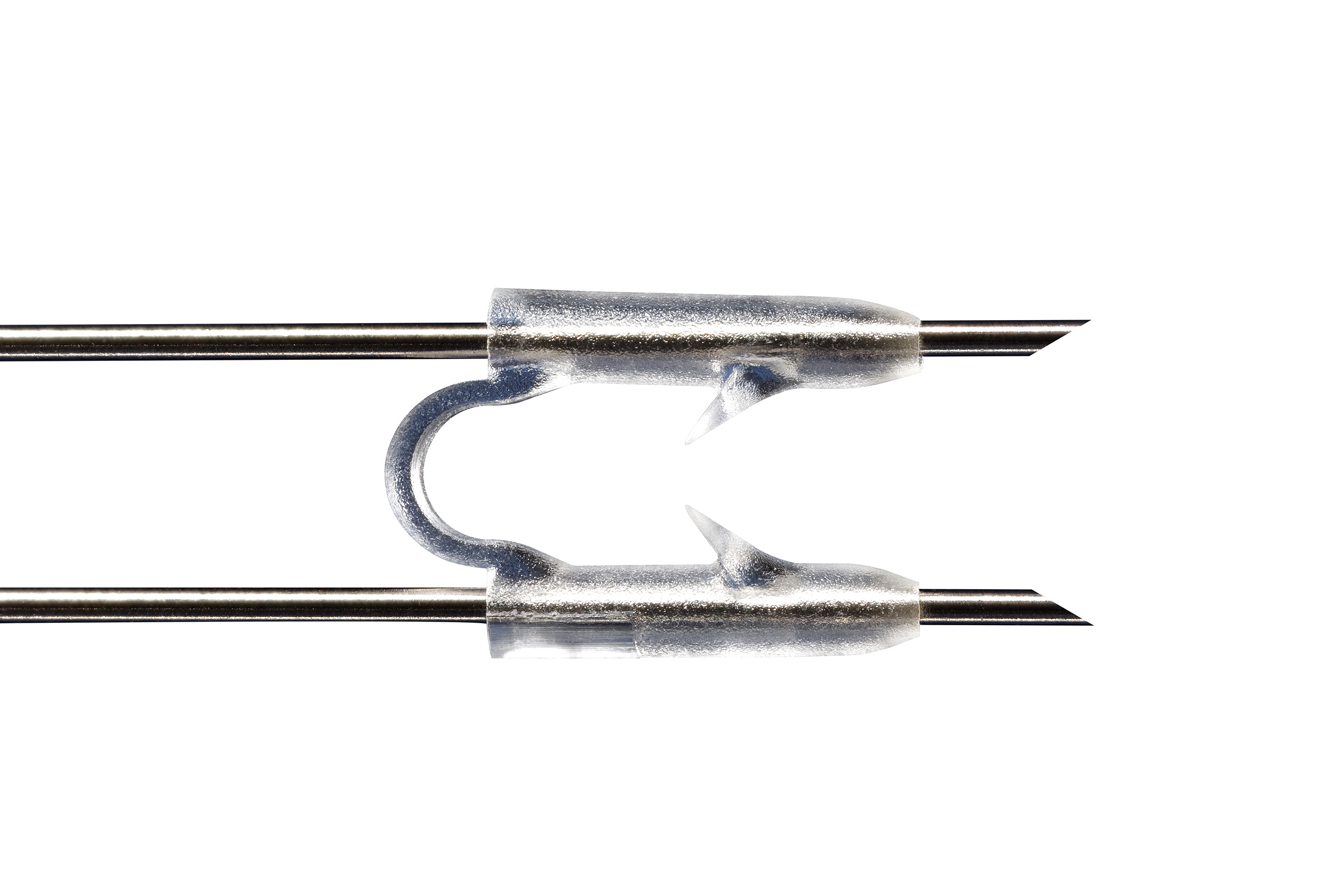
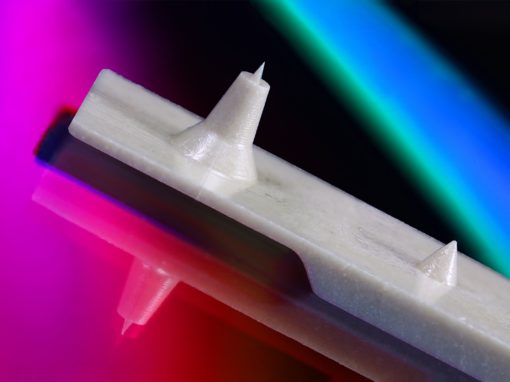
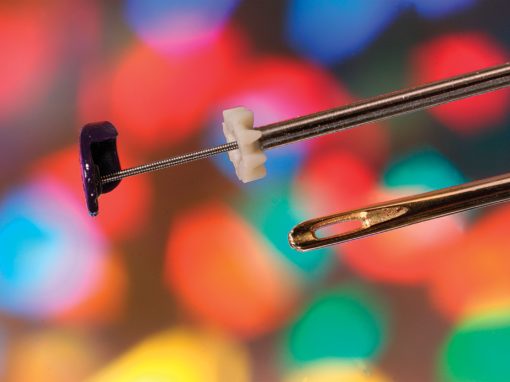
Quality You Can See
It is with MTD’s specialized process that micro-sized parts and features, micro-geometry, and tight tolerances of <.0005″ (0.0127mm) can be achieved. Our quality testing exceeds that of any other firm in our field. In fact, our standards are usually above and beyond our customers’ requirements. In an industry where precision is paramount, integrated inspection systems allow us to trace to the nth degree, leaving no doubt about ensuring the success of our customers’ products.